RESEARCH: SPECTROSCOPY
Reflectance spectroscopy, a portion of solar system astronomy, is vital for uncovering the chemical composition, temperature, and physical states of celestial bodies and their atmospheres by analyzing the light they emit or reflect. This technique provides detailed insights into planetary processes, surface compositions, and potential indicators of habitability or past conditions favorable to life.
To understand the research we pursue at Florida Space Institute in the context of reflectance spectroscopy, it is important to know the fundamentals of it.
WHAT IS SPECTROSCOPY?
reflectance spectroscopy is a technique used to study how materials interact with light, based on the principle that different substances emit or absorb light at different wavelengths, depending on factors like temperature and composition. By examining the light’s spectrum—its unique pattern of colors—scientists can determine various properties of the material, such as its temperature and the elements or compounds it contains, without needing to physically sample it.
The first step in reflectance spectroscopy involves separating light into its individual wavelengths to produce a spectrum. This can be achieved using devices like a glass prism, a diffraction grating, or a combination of the two, known as a grism. Natural spectra, like rainbows, occur when sunlight passes through water droplets, which act like prisms. Specialized instruments called spectroscopes and spectrographs are used to capture and measure spectra in more detail.
A spectrum can be shown as an image, but to analyze it precisely, scientists typically plot it on a graph. This allows for the detection of subtle variations in brightness and wavelength that may be too faint for the human eye to perceive.
There are different types of spectra, each providing distinct information. A continuous spectrum shows a smooth transition of brightness across the colors without gaps. The brightness typically varies evenly, and one example of a continuous spectrum is the blackbody curve. This spectrum represents the range of colors emitted by an object based on its temperature. For instance, hot objects like stars emit more blue light, making them appear bluer, while cooler objects emit more red light, appearing redder.
Absorption spectra occur when certain wavelengths of light are absorbed by atoms or molecules in a material, creating dark lines (called absorption lines) in an otherwise continuous spectrum. A transmission spectrum is a type of absorption spectrum, occurring when light passes through a material, like a planet’s atmosphere. In this case, some light is absorbed, and some is transmitted, with the absorption patterns providing information about the atoms and molecules in the atmosphere.
What’s particularly useful about these spectra is that each element or compound has a unique pattern of absorption lines, known as its “signature.” By identifying these lines, scientists can determine the elements or compounds present in a material, as well as its temperature, density, and other characteristics. This is possible because each element’s electrons absorb light at specific wavelengths when they transition between energy levels.
*This information was sourced from the pdf attached here, source material credits to the original author.
Here are some of the main components we use research in reflectance spectroscopy to further understand celestial phenomena
MODELING AND INTERPRETATION

DATA ACQUISITION
CALIBRATIONS
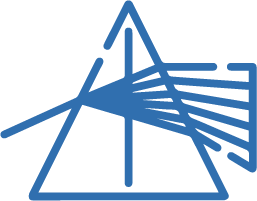
SPECTRAL ANALYSIS
Every astronomical object has a unique spectrum, or “rainbow fingerprint”, that allows astronomers to determine its contents, age, formation history, movements through space, temperature and more!